HyQvia is a liquid medicine that is given under the skin (subcutaneously) to treat primary immunodeficiency (PI) in people 2 years and older.

That’s right, infuse every 3-4 weeks!
Meet the only monthly* subQ IG treatment.
HyQvia® [Immune Globulin Infusion 10% (Human) with Recombinant Human Hyaluronidase] is a subcutaneous, or subQ, immune globulin (IG) treatment for primary immunodeficiency, or PI.
If you’re reading this, you’re likely facing PI in one way or another, whether that’s a diagnosis for yourself, a family member, or a friend. Read on to learn what makes HyQvia different from other therapies.
*Every 3 or 4 weeks.
What makes HyQvia different?
Monthly* infusions
Depending on the infusion schedule you and your doctor work out, there’s a possibility you can go up to 28 days between infusions, unlike other subQ therapies, where weekly or even daily infusions are necessary.
*Every 3 or 4 weeks.
Infusion: About 2 hours
Similar to intravenous immunoglobulin (IVIG), HyQvia can be infused once monthly.* However, unlike other IVIG infusions, which can take 2-4 hours (or sometimes up to 6 hours, including travel time to and from an infusion center), HyQvia can be infused in about 2 hours and given at home, with proper training from a nurse or HCP.
Hyaluronidase (Hy)
Hy is something found naturally in your body. It is a component of HyQvia. Infused first, it allows more IG to be absorbed into your bloodstream.
In the clinical trials, there were no observable changes in the skin or subQ tissue in almost 3,000 infusions and maximum exposure of over 3 years. After a HyQvia infusion, a temporary, soft swelling may occur around the infusion site, which may last 1 to 3 days due to the volume of fluid infused. The effect of Hy is reversible and your skin will be restored within 24 to 48 hours following your infusion.
It’s important to understand that HyQvia is not right for everyone. Do not take it if you are allergic to IgG, hyaluronidase, other blood products, or any ingredient in HyQvia. Talk to your doctor about any concerns you may have before starting HyQvia or at any time during your treatment. Learn more about HyQvia safety.
Own your infusion experience with flexible administration or infusion options.
Not everything is one size fits all, especially when it comes to something as important as your PI treatment.
You choose whether you want to infuse in the comfort of your own home by yourself after adequate training from a healthcare professional, or at an infusion center with a nurse. You’ll need to check with your insurance carrier to see what’s covered, but you have options.
Places where infusions are completed:
At home†
Infusion center
Doctor’s office
Hospital
†After proper training from a nurse or HCP.
People who can help administer your infusions:
You
Family or friend
Healthcare professional
Visual learner? We’ve got you.
Watch this short video to learn more about HyQvia and how it may help you or a loved one treat PI.
If you'd rather read about HyQvia for yourself, we get it.
How HyQvia Works
Hyaluronidase, or Hy, is what allows you to infuse IG monthly.*
Hy allows a larger amount of IG to be absorbed, which makes monthly* infusions possible.
Hy is in the smaller vial of your dual-vial unit, and it’s infused first. It allows your body to absorb more IG just under the skin. That’s what we mean by subcutaneous—it’s an alternative to having an IV give medicine directly into a vein. Hy is something that’s found naturally in your body, and HyQvia uses it to help infuse the IG you need. In the clinical trials, there were no observable changes in the skin or subQ tissue in almost 3,000 infusions and maximum exposure of over 3 years. After a HyQvia infusion, a temporary, soft swelling may occur around the infusion site, which may last 1 to 3 days due to the volume of fluid infused. The effect of Hy is reversible and your skin will be restored within 24 to 48 hours following your infusion. Learn about additional side effects that can occur with HyQvia.
*Every 3 or 4 weeks.
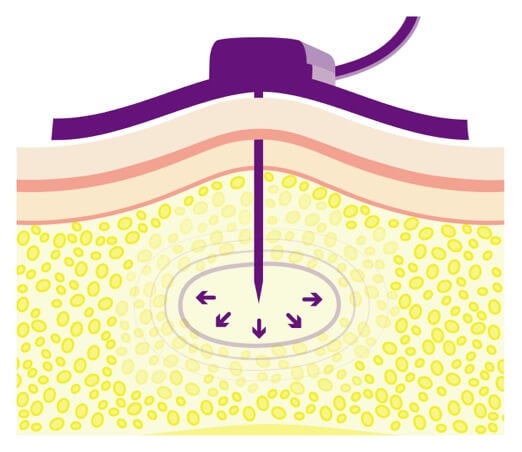
The body naturally contains hyaluronan.
The subQ tissue directly beneath your skin is naturally filled with hyaluronan.
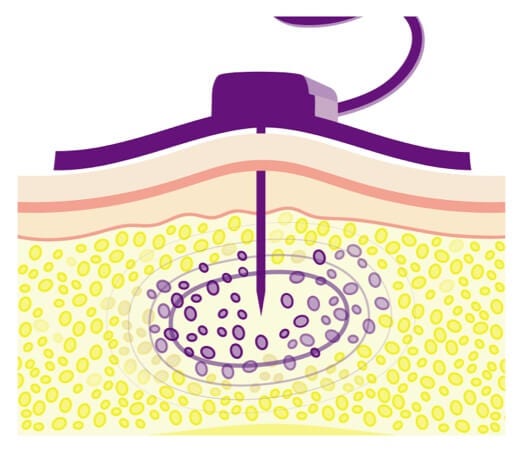
Hyaluronan limits the volume of IG that can be infused.
Without the Recombinant Human Hyaluronidase (Hy) in HyQvia, hyaluronan would limit the amount of IG that can be infused into the subQ tissue.
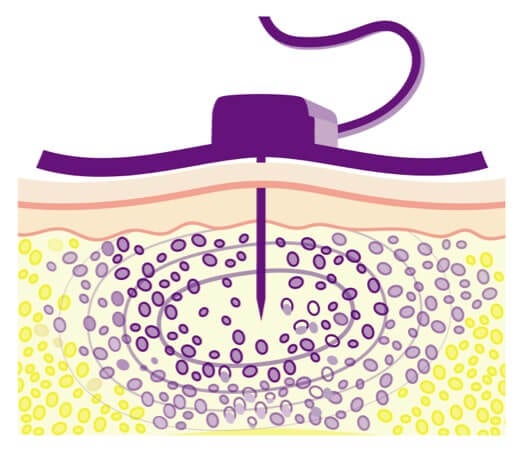
Hy makes room for a larger volume of IG.
Hy allows a larger amount of IG to reach the subQ tissue and be absorbed into the bloodstream. That’s why HyQvia can be infused less frequently than other subQ IG treatments.

HyQvia vs IVIG
The bottom line is that HyQvia is infused subcutaneously and not intravenously.
This means there is a shorter needle, so it’s less invasive. You get to keep the schedule of an IVIG therapy (every 3 to 4 weeks) with the option of infusing in the comfort of your own home, after adequate training from a healthcare professional. When you infuse into a vein, you have to travel to and from an infusion center so a nurse can complete your infusion. A HyQvia infusion takes about 2 hours, and an IV infusion takes 2-4 hours, sometimes even up to 6 hours.
Less total time infusing
The average time for each infusion was 2.08 hours in the clinical trial. IVIG infusions can take 2-4, sometimes even up to 6 hours, including travel time.
Smaller needles
Because HyQvia is NOT given into a vein, there’s no need for an IV needle. The needle to infuse subcutaneously is smaller.
Flexible infusion options
You choose whether you want to infuse in the comfort of your own home (with training), at an infusion center, by yourself, or with a nurse. Check with your insurance carrier to see what's covered.