HyQvia is a liquid medicine that is given under the skin (subcutaneously) to treat primary immunodeficiency (PI) in people 2 years and older.

Do you know about subQ?
HyQvia is infused subcutaneously, or the subQ way.
That means it’s given just under your skin, not into a vein.
There are two different ways to infuse HyQvia subcutaneously. You can infuse with a peristaltic pump, or you can infuse with a syringe driver pump. You’ll talk with your doctor about the best way for you to infuse, and the specialty pharmacy and nurse who trains you can talk you through any specific questions you may have, including questions about the pump you’ll be using.
When starting to infuse, there’s a ramp-up period.
This means that you’ll start slowly and gradually build up to your prescribed dose since it’s new to your body. Your ramp-up schedule will be given to you by your doctor and will, over time, get you to your full prescribed dose. You’ll eventually shift from 1 infusion a week to 1 infusion every 3 or 4 weeks, depending on the frequency your doctor prescribes. Your doctor will decide if any dose adjustments need to be made, as necessary, based on your clinical response.
Ramp-up example for 30-gram dose of HyQvia with a 4-week treatment interval*
The first dose of HyQvia is given 1 week after the last infusion of the patient’s previous IG treatment.
Here’s how an infusion site typically looks before and after infusing.
Knowing what to expect will hopefully make starting your infusions less stressful. Everyone’s infusion experience is different. However, it’s not uncommon to experience a temporary, soft swelling at the infusion site. You may hear someone refer to this as a “pancake” because that’s what it looks like. It happens because of the volume of fluid infused and may last anywhere from 1 to 3 days after your infusion. For some, it’s gone the next day.
Beginning of infusion
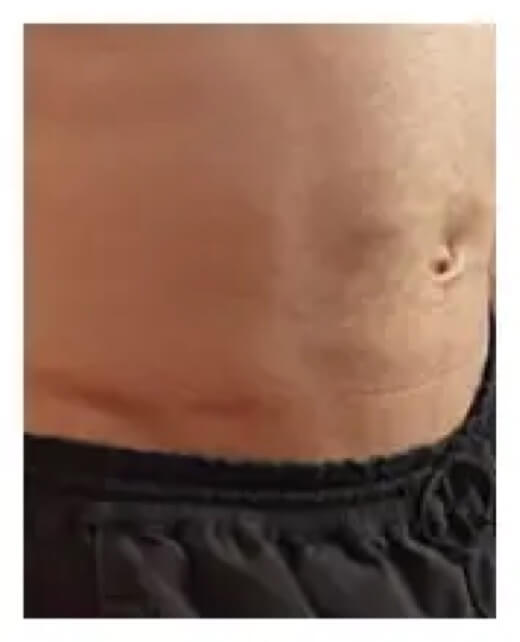
Patient weight: 168 lb Volume: 500 mL
End of infusion
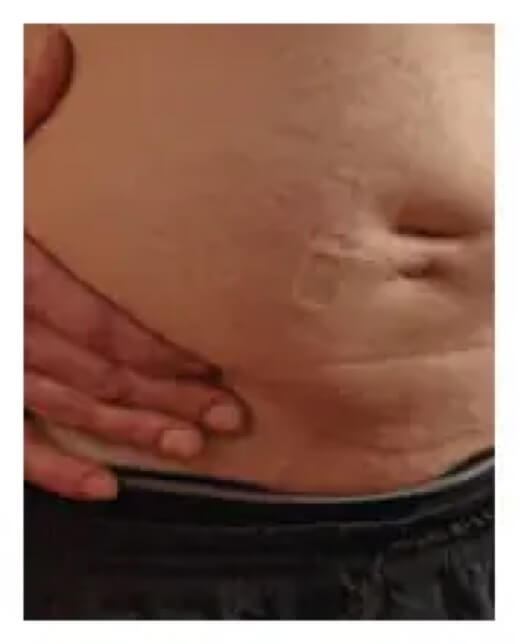
Results in diffuse, pancake-like swelling
24 hours after infusion
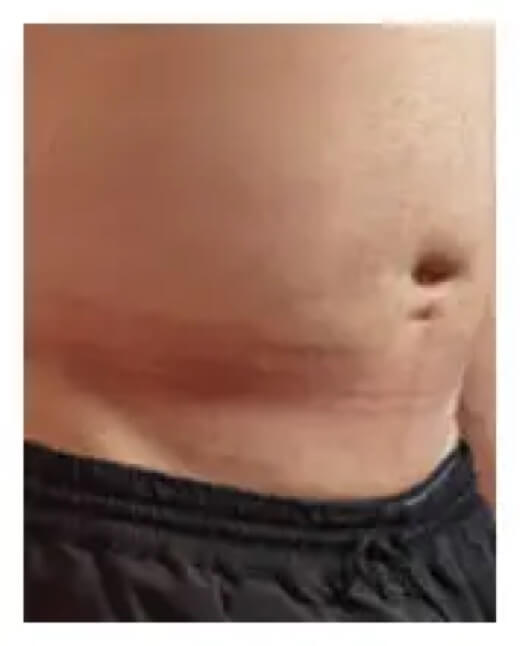
Infusion site swelling generally resolved within 1 to 3 days
What the infusion experience is like.
Before the infusion:
- Get comfy
- Get your supplies out and ready
- Read over the infusion steps if you need to, especially if you're just starting out
- If appropriate, make sure you're hydrated before infusing and have a drink nearby in case you're thirsty during your infusion
- If others are around, let them know your infusion time so there's as little disruption for you as possible
- Always follow the directions provided by your doctor regarding your dose and schedule
During the infusion:
- Try your best to relax and stay comfy, as the average infusion time is about 2 hours. Read, play a game, catch up on your fave show, call a friend, get creative and use this time for you
- You may experience mild to moderate pain, redness, swelling and itching (BUT, these are common and generally go away within a few hours). These aren't all of the possible side effects, but ones you may immediately notice
- In addition to local infusion site reactions, the most common side effects may include headache, nausea, fatigue, diarrhea, fever, and vomiting
- These are not all the possible side effects. Talk to your doctor about any side effect that bothers you or that does not go away. If side effects increase in severity or persist more than a few days, call your doctor or hospital emergency services immediately
After the infusion:
- If appropriate, continue to drink fluids to stay hydrated
- Record your infusion details and any reactions or notes for yourself or your doctor. Make sure to inform your doctor about any side effect that bothers you or does not go away
- A temporary, soft swelling, referred to as a pancake (yep, a pancake), may occur at your infusion site. It's named for the shape it takes and is pretty common. It can last 1 to 3 days due to the volume of fluid infused but will subside as your medicine is absorbed
INFUSING WITH PERISTALTIC PUMP
Peristaltic pumps
This pump helps deliver your prescribed dose, at an infusion rate you and your doctor are comfortable with.
Want to see for yourself? Watch the video. Would you rather read more? Get the brochure.
- 0:00 Get familiar with hy5
- 3:35 hy1: Get ready
- 4:31 hy2: Prepare the hyaluronidase, or Hy
- 6:42 hy3: Prepare the immune globulin, or IG
- 10:37 hy4: Infuse
- 15:30 hy5: Finish up
INFUSING WITH SYRINGE DRIVER PUMP
Syringe Driver Pump
Download the brochure to learn more.
